
August 1, 2022
Inner Circle
The Difference Between PVC and PE
Contributed by Josh Goldberg, Business Development Manager
We are brought up in a world surrounded by plastics, but why are there so many of them, and what is the difference among them? To a polymer chemist, there is an entire field of exciting materials based on what properties are essential for a given job. However, the rest of the world sees plastic as just plastic. To address this point, we can ask: what is a polymer and how does chemistry fit into this discussion?
A polymer is a macromolecule composed of several repeating units called a monomer (mono meaning one). The mixing, cross-linking, and molecule size determine the type of properties a polymer has and how it is manufactured. Examples of polymers include polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), polyvinylidene fluoride (PVDF), rubber, inks/paints, adhesives, proteins, nucleic acids, and the list goes on and on. In short, a polymer covers a vast spectrum of materials. When we think of plastics, we tend to think of things like water bottles, Tupperware®, cling wrap, shower curtains, and inexpensively-made parts, not polymers like, PE, PP, PVC, and PVDF. While the plastic items mentioned above are made from polymers like PE, PP, and PVC, there is a vast difference between these inexpensively-manufactured plastic items and performance polymers like PE, PP, and PVC used for piping systems and valves for industrial plumbing applications. So, how are these two categories of plastics different?
Polymers generally fall into two different groups: thermoplastics and thermosets. A thermoplastic is a jumbled tangle of polymers, and a thermoset is a jumbled tangle of polymers that are cross-linked to each other. Both types of polymers come with their own set of advantages and disadvantages. For example, thermoplastics can be remolded, weldable, and recyclable. However, they have a higher comparative coefficient of thermal expansion (CTE), and can be more susceptible to UV and harsh chemical attacks when compared to thermosets (with the exception of fluoropolymers like PVDF and ECTFE). For this discussion, we are going to focus on thermoplastics, like PE, PP, and PVC, and what sets them apart from each other and their “cheaper cousins” that we all associate with the word plastics.
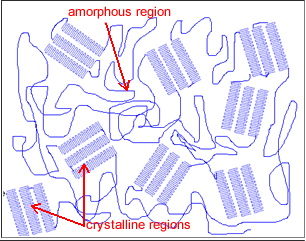
Thermoplastics, like PE, PP, and PVC, can be made quickly and inexpensively and are usually mixed with some plasticizer to make them pliable and long-lasting. So how can we make these polymers so strong that they can withstand chemicals, like sulfuric acid, peroxides, caustics, or bleach? The answer lies in using the polymer structure to manipulate the polymer’s properties. There are two general parts to a polymer: the crystalline phase and the amorphous phase. What does each stage do, and how can that be used to the polymer’s advantage?
The crystalline phase of a polymer can take on many shapes, but it generally appears like a fuzzy ball or nodule called the crystalline region. If we look closer at this region, we will see that it is composed of little crystallites. If there were access to a microscope strong enough to look at the crystallites, we would see that they are made up of the polymer folding back and forth on itself in a regular sequence line like an accordion fold in a piece of paper. This regular back and forth folding of the polymer is key to some fundamental physical properties of a polymer.
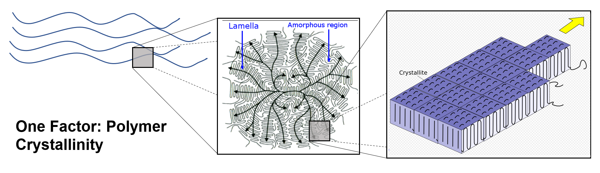
The regular shape means that it takes more energy to break up the crystalline group so that the crystalline region can determine the polymer’s melt temperature (Tm). The tight regular shape takes more energy to pull apart, so the crystalline region can also determine properties like tensile strength. Since the polymer is folded against itself like a paper accordion, most of the polymer is safely tucked away against itself, leaving fewer sites along the polymer chain open to chemical or UV attack. Because of this crystalline region, polymers also have properties associated with chemical resistance. However, it is the size, shape, and distribution of the crystalline region through the bulk polymer that determines the polymer’s degree of strength and its chemical and heat resistance. These key attributes are what determine many physical properties of materials like PE and PP. So why don’t we make a super-strong polymer that is all crystalline and can withstand high temperatures and the worst chemicals? In material science, there are always trade-offs in material properties, so let’s take a quick look at PVC to demonstrate this point.
The polymer PVC is very attracted to itself. This can be a good thing because it forms very strong crystals, giving PVC great heat and chemical resistance and a good amount of tensile strength. However, since PVC is very attracted to itself, which causes its crystals to grow bigger over time, this, in turn, can create cracking of the bulk polymer and create brittleness. To prevent this, plasticizers are added to get in between the polymer chains to prevent crystal formation. It is worth mentioning that something can be brittle and have high tensile strength and hardness simultaneously. Brittleness reflects the material’s ability to flex when force is applied to it without shattering, hardness demonstrates the material’s ability to be scratched, and tensile strength refers to the force it takes to pull a material apart. So, while the hardness and tensile strength would be elevated over the material’s starting properties, the material would also shatter when force is applied. This leads to the other important property of a polymer: the amorphous phase.

The amorphous phase is the chaotic polymer region around the crystalline regions. When enough heat energy is introduced into the polymer and the region becomes mobile, this is known as the glass-transition temperature (Tg). The advantage to having a material with a low Tg where the amorphous phase is mobile at room temperature, like in PE and PP, is that this imparts a great amount of elasticity to the material. This is why you can whack PE and PP pipes with a sledgehammer and it doesn’t crack or break. When the amorphous phase is mobile, it can act springy and absorb a great amount of energy into the material without breaking the material. If the same thing were done to PVC, it would shatter into hundreds of little pieces since its amorphous phase is frozen in place as indicated by its high Tg.
By manipulating the crystalline region and using the amorphous region to their advantage, materials like PE and PP can gain tensile strength, temperature, and chemical resistance while maintaining flexibility. These are known as performance polymers.
Asahi/America’s Chem Proline® Advanced PE (PE100RC) Piping System or Proline® PP-R Piping System are known as performance polymers, which were transformed from humble plastics to high-performance polymeric materials.
Although these performance polymers, like PE, PP, and PVC have great chemical and temperature resistance, they are not capable of handling nasty chemicals, like concentrated sulfuric acid >85%. There is a particular group of polymers that we will cover in a follow-up article, called fluoropolymers, that can handle sulfuric acid and other highly reactive chemicals and high temperatures.
Please contact me at jgoldberg@asahi-america.com or visit our website if you would like to sign up for a lunch & learn where you can learn more about the world of polymers and Asahi/America’s high-performance valve and piping systems.
EDITOR’S NOTICE: Please note, the information in this article is for educational purposes only and does not supersede any Asahi/America technical information or product specifications. Please consult Asahi/America’s technical department at 1-800-343-3618 or pipe@asahi-america.com on all product applications in regards to material selection based on the pressure, temperature, environmental factors, chemical, media, application, and more.
